Tech Tip
During enhanced reductive dechlorination (ERD), naturally occurring sulfate (SO4 2-) is reduced to hydrogen sulfide (H2S). High concentrations of H2S can inhibit reductive dechlorination, reducing remediation efficacy (Hoelen and Reinhard, 2004). Sung (2005) found that 64 mg/L total sulfide inhibited growth of Desulfuromonas michiganensis strain BB1, Sulfurospirillum multivorans, Desulfitobacterium sp. strain Viet1, and Dehalococcoides sp. strain FL2 and strain BAV1. However, at low concentrations (<16 mg/L), sulfide does not inhibit dechlorinators (Löffler et al., 2005).
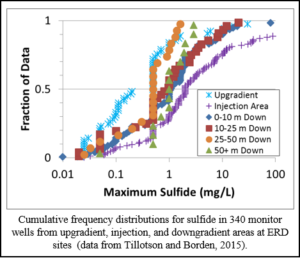
In most aquifers, substantial amounts of naturally occurring iron are present and H2S will rapidly precipitate as insoluble iron sulfide minerals (FeS or FeS2; Cozzarelli et al., 1999). However, when sulfate concentrations are high (> 1,000 mg/L) and the aquifer material contains little or no solid phase iron, dissolved sulfide concentrations can reach inhibitory levels. The graph shows cumulative frequency distributions for maximum observed sulfide concentrations in 340 monitor wells at ERD sites surveyed by Tillotson and Borden (2015). The data show that maximum sulfide concentrations observed in some injection-area wells were higher than in upgradient wells, indicating sulfate reduction is leading to some increase in sulfide. However, maximum sulfide concentrations in the injection area were typically less than 10 mg/L and decreased with distance downgradient, indicating sulfide production is not expected to be an issue at most sites.
For more information on sulfide production, check out the article on Secondary Water Quality Impacts of Anaerobic Bioremediation on ENVIRO.wiki.
