Tech Tip
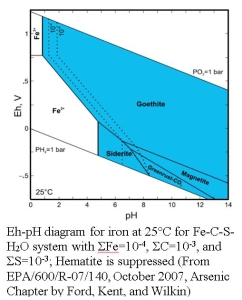
Eh or reduction potential can be used to evaluate subsurface geochemical conditions by comparing measured values to published values of Eh for different reactions or used in combination for pH measurements to identify stability field of different species (see Eh‐pH diagram for iron below).
 Oxidation‐Reduction Potential (ORP) is a qualitative indication of the relative oxidizing or reducing condition of groundwater and is typically monitored using a handheld voltage meter that measures the electrical potential between a platinum electrode and a reference electrode. In most cases, the platinum electrode and reference electrode are combined into a single probe that is inserted into the solution. Positive values of ORP indicate generally oxidizing environment while negative values indicate a reducing environment.
Oxidation‐Reduction Potential (ORP) is a qualitative indication of the relative oxidizing or reducing condition of groundwater and is typically monitored using a handheld voltage meter that measures the electrical potential between a platinum electrode and a reference electrode. In most cases, the platinum electrode and reference electrode are combined into a single probe that is inserted into the solution. Positive values of ORP indicate generally oxidizing environment while negative values indicate a reducing environment.
In theory, field ORP readings can be converted to Eh by correcting for the electrode potential of the reference electrode (Eref). Eref values for common Ag/AgCl reference electrodes vary from +236 mV for 1 M KCl to +197 mV for saturated KCl solutions (Nordstrom and Wilde, 2005). For example, assume ORP was measured in the field with a platinum ‐ Ag/AgCl electrode containing 1 M KCl and the meter reading was 200 mV. The Eh of the solution would be:
Eh = 200 mV + 236 mV = 436 mV = 0.44 V
In practice, a variety of factors plague ORP measurements including effects of solution temperature and pH, irreversible and slow reaction, multiple redox couples, and electrode poisoning. As a result, field measured ORP values should be considered as a rough qualitative indicator of the system Eh.
