Tech Tip
Why can’t I control the pH of my aquifer? pH and Acidity are not the same Acidity is the amount of base required to neutralize acids present in a water sample. This is important because it determines the amount of buffer required to adjust aquifer pH.
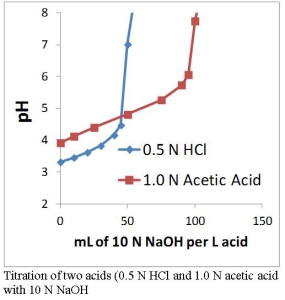
Since acids can disassociate to different extents, two solutions with the same pH can have different acidities. The figure to the right shows the pH of solutions containing 0.5 N HCl and 1.0 N acetic acid. The initial pH of the acetic acid solution is higher than that of the HCl solution, even though the acetic acid solution has a higher acidity.
Acidity is measured by Standard Method 2310 where a water sample is titrated to a specified end point with a strong base. Traditionally, an end point pH of 3.7 is used for strong acids (methyl orange acidity) and an end point pH of 8.3 is used for weak acids (phenolphthalein or total acidity) such as dissolved CO2.
In some aquifers, the pH is low due to high concentrations of dissolved CO2. Below the water table, the high pressure prevents degassing. However, when the sample is brought to the surface, the lower pressure results in CO2 degassing. If this occurs, pH will increase and the measured acidity will not be representative of in situ conditions.